How does cancer spread in the body? Cancer metastasis may be driven by a protein on the move
Researchers discovered that when cells are under stress, a key protein can travel to the nucleus and reprogram cells to migrate and become more invasive.
By Zara Abrams
A surprising finding from Keck School of Medicine of the University of Southern California (USC) reveals key details about how cancer cells metastasize and suggests new therapeutic approaches for halting their spread.
The research, supported by the National Institutes of Health, centers on a cellular chaperone protein known as GRP78, which helps regulate the folding of other proteins inside cells. Previous studies from the same team, led by Amy S. Lee, PhD, professor of biochemistry and molecular medicine at the Keck School of Medicine of USC, have shown that when cells are under stress (due to COVID-19 or cancer), GRP78 gets hijacked, allowing viral invaders to replicate, and cancers to grow and resist treatment.
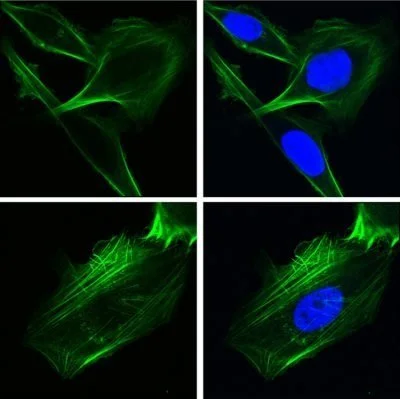
Researchers from the Keck School of Medicine of USC used imaging techniques to study how the protein GRP78 controls cancer cell behavior. In the top row, human lung cancer cells were engineered to over-express GRP78 in the nucleus. In the bottom row, cells lacked GRP78 in the nucleus. The green staining depicts the cytoskeletal protein F-actin which controls cell shape and motility and the blue staining depicts the nucleus. Image courtesy of Ze Liu, Ph.D, and Amy Lee, PhD
Lee and her colleagues have now made an unexpected discovery that may eventually enable scientists to protect cells from that hostile takeover. Typically, GRP78 resides in a part of the cell called the endoplasmic reticulum. But when cells are under stress, the chaperone protein migrates to the cell’s nucleus, where it alters gene activities and changes the behavior of the cell, allowing the cancer cells to become more mobile and invasive.
“Seeing GRP78 in the nucleus controlling gene expression is a total surprise,” said Lee, the study’s senior author and the Judy and Larry Freeman Chair in Basic Science research at the USC Norris Comprehensive Cancer Center. “When it comes to the basic mechanisms of cancer cells, this is something novel that, to my knowledge, no one has observed before.”
The findings, just published in the Proceedings of the National Academy of Sciences, could represent a paradigm shift for cell biology, and have implications for cancer therapeutics research, Lee said.
An unanticipated discovery
The new discovery started as an incidental one. Ze Liu, PhD, a postdoctoral researcher in Lee’s lab and the study’s first author, was analyzing how GRP78 regulates a gene known as EGFR, long linked to cancer. He noticed something surprising: GRP78 controls the gene activity of EGFR, raising the intriguing possibility that GRP78 may have entered the nucleus and assumed a new role. But the chaperone protein was long thought to exist primarily in the endoplasmic reticulum of cells.
To confirm their hypothesis, Liu, Lee and their colleagues used confocal microscopy, which offers high-resolution 2D and 3D imaging, coupled with an advanced technique for capturing images of live cells, to directly observe GRP78 in the nucleus of lung cancer cells, as well as normal cells under stress. They then used several other techniques, including biochemical analysis and mRNA knock-down of GRP78. These techniques allowed them to identify the signal within GRP78 that enables it to enter the nucleus and confirm that when GRP78 is present in the nucleus, it stimulates EGFR gene activity.
Next, the researchers set out to learn more about what happens in a cell after GRP78 enters the nucleus. Using a sophisticated form of RNA sequencing they compared lung cancer cells engineered to over-express GRP78 in the nucleus to cells lacking GRP78 in the nucleus in order to learn which genes were affected.
“To our big surprise, we found that the key genes being regulated by GRP78 in the nucleus are mainly involved with cell migration and invasion,” Lee said.
The team found that GRP78 binds to ID2, another cellular protein. ID2 typically suppresses genes (including EGFR), many of which allow cells to migrate. But when bound to GRP78, ID2 can no longer do its job. Without that suppression, cancer cells become more invasive.
Lung cancer cells growing in culture. Image: Lung cancer cells., Anne Weston/Francis Crick Institute
Broad implications for cancer and cell biology
The new findings point to several potential new approaches for cancer treatment, including down-regulating the activity of GPR78 to suppress EGFR in lung cancer, or preventing it from binding to ID2. GRP78 could also bind to other proteins in the nucleus critical for cancer, opening up a new line of research in cancer biology. While the present study analyzed lung cancer cells, GRP78 plays a similar role in various types of cancers, including pancreatic, breast and colon cancer.
The discovery that GRP78, a major endoplasmic reticulum protein, can travel to the nucleus and assume new functions, could also have broad implications across the field of cell biology. Lee said it’s possible—even likely—that other proteins that typically reside in one part of the cell could, under stress or other triggers, migrate to another part of the cell and alter cell behavior in multiple ways.
“This is a new concept,” she said. “The protein itself is the soldier that does the job, but now we’re thinking it’s not just about the soldier, but also where the solider is deployed.”
Lee and her team are also studying drugs that can inhibit the expression or activity of GRP78. An ongoing study of theirs suggests that small molecules that inhibit GRP78, such as YUM70, may even be able to block GRP78 activity in the nucleus of cells.
Provided by Keck School of Medicine of USC
Reference: Ze Liu, Guanlin Liu, Dat P. Ha and Amy S. Lee. ER chaperone GRP78/BiP translocates to the nucleus under stress and acts as a transcriptional regulator. PNAS (2023). https://doi.org/10.1073/pnas.2303448120