Decoding the complexity of Alzheimer’s disease
By analyzing epigenomic and gene expression changes that occur in Alzheimer’s disease, researchers identify cellular pathways that could become new drug targets.
Anne Trafton / MIT News
Credit: Conceptual image of a person with Alzheimer's disease generated by DALL-E 2 from Microsoft Bing.
Alzheimer’s disease affects more than 6 million people in the United States [in Spain, 1,2 million] and there are very few FDA-approved treatments that can slow the progression of the disease.
In hopes of discovering new targets for potential Alzheimer’s treatments, MIT researchers have performed the broadest analysis yet of the genomic, epigenomic, and transcriptomic changes that occur in every cell type in the brains of Alzheimer’s patients.
Using more than 2 million cells from more than 400 postmortem brain samples, the researchers analyzed how gene expression is disrupted as Alzheimer’s progresses. They also tracked changes in cells’ epigenomic modifications, which help to determine which genes are turned on or off in a particular cell. Together, these approaches offer the most detailed picture yet of the genetic and molecular underpinnings of Alzheimer’s.
The researchers report their findings in a set of four papers appearing in Cell. The studies were led by Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory, and Manolis Kellis, a professor of computer science in MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) and a member of the Broad Institute of MIT and Harvard.
“What we set out to do was blend together our computational and our biological expertise and take an unbiased look at Alzheimer’s at an unprecedented scale across hundreds of individuals — something that has just never been undertaken before,” Kellis says.
A complex interplay.
The findings suggest that an interplay of genetic and epigenetic changes feed on each other to drive the pathological manifestations of the disease.
“It’s a multifactorial process,” Tsai says. “These papers together use different approaches that point to a converging picture of Alzheimer’s disease where the affected neurons have defects in their 3D genome, and that is causal to a lot of the disease phenotypes we see.”
Many efforts to develop drugs for Alzheimer’s disease have focused on the amyloid plaques that develop in patients’ brains. In their new set of studies, the MIT team sought to uncover other possible approaches by analyzing the molecular drivers of the disease, the cell types that are the most vulnerable, and the underlying biological pathways that drive neurodegeneration.
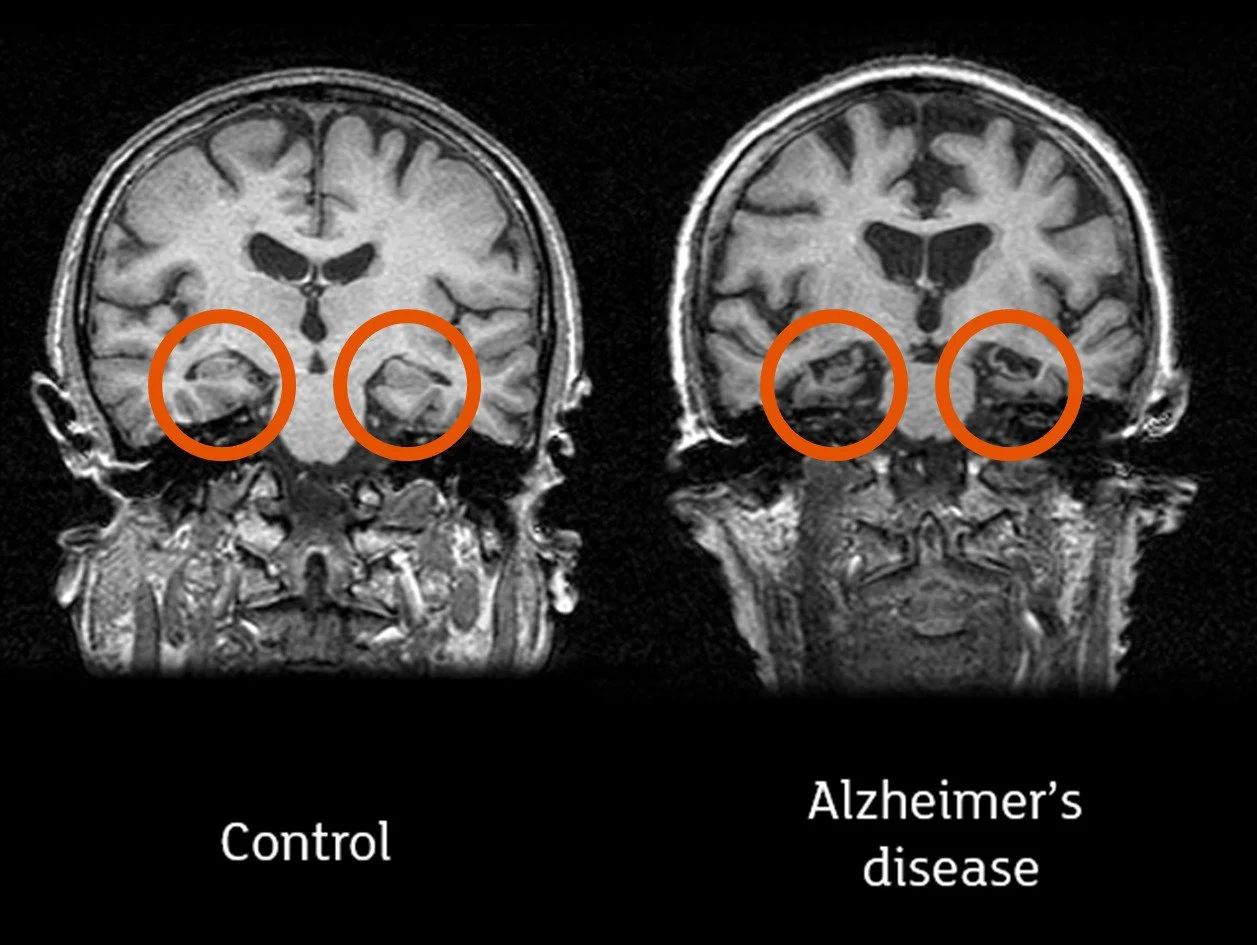
In Alzheimer’s disease the hippocampi (circled in the image) are often affected first. Credit: Professor John O’Brien, University of Cambridge and Newcastle University
To that end, the researchers performed transcriptomic and epigenomic analyses on 427 brain samples from the Religious Orders Study/Memory and Aging Project (ROSMAP), a longitudinal study that has tracked memory, motor, and other age-related changes in older people since 1994. These samples included 146 people with no cognitive impairment, 102 with mild cognitive impairment, and 144 diagnosed with Alzheimer’s-linked dementia.
In the first Cell paper, focused on gene expression changes, the researchers used single-cell RNA-sequencing to analyze the gene expression patterns of 54 types of brain cells from these samples, and identified cellular functions that were most affected in Alzheimer’s patients. Among the most prominent, they found impairments in the expression of genes involved in mitochondrial function, synaptic signaling, and protein complexes needed to maintain the structural integrity of the genome.
This gene expression study, which was led by former MIT postdoc Hansruedi Mathys, graduate student Zhuyu (Verna) Peng, and former graduate student Carles Boix, also found that genetic pathways related to lipid metabolism were highly disrupted. In work published in Nature last year, the Tsai and Kellis labs showed that the strongest genetic risk for Alzheimer’s, called APOE4, interferes with normal lipid metabolism, which can then lead to defects in many other cell processes.
Epigenomics.
In the study led by Mathys, the researchers also compared gene expression patterns in people who showed cognitive impairments and those who did not, including some who remained sharp despite having some degree of amyloid buildup in the brain, a phenomenon known as cognitive resilience. That analysis revealed that cognitively resilient people had larger populations of two subsets of inhibitory neurons in the prefrontal cortex. In people with Alzheimer’s-linked dementia, those cells appear to be more vulnerable to neurodegeneration and cell death.
“This revelation suggests that specific inhibitory neuron populations might hold the key to maintaining cognitive function even in the presence of Alzheimer’s pathology,” Mathys says. “Our study pinpoints these specific inhibitory neuron subtypes as a crucial target for future research and has the potential to facilitate the development of therapeutic interventions aimed at preserving cognitive abilities in aging populations.”
In the second Cell paper, led by former MIT postdoc Xushen Xiong, graduate student Benjamin James, and former graduate student Carles Boix PhD ’22, the researchers examined some of the epigenomic changes that occurred in 92 people, including 48 healthy individuals and 44 with early or late-stage Alzheimer’s. Epigenomic changes are alterations in the chemical modifications or packaging of DNA that affect the usage of a particular gene within a given cell.
Simple test could help predict risk of Alzheimer’s disease 20 years in advance. ANU School of Physics researcher Professor Patrick Kluth looks at a very thin membrane with tiny pores in it that filters proteins in our blood that show early neurodegeneration. Credit: Jack Fox / ANU
To measure those changes, the researchers used a technique called ATAC-Seq, which measures the accessibility of sites across the genome at single-cell resolution. By combining this data with single-cell RNA-sequencing data, the researchers were able to link information about how much a gene is expressed with data on how accessible that gene is. They could also start to group genes into regulatory circuits that control specific cell functions such as synaptic communication — the primary way that neurons transmit messages throughout the brain.
Using this approach, the researchers were able to track changes in gene expression and epigenomic accessibility that occur in genes that have previously been linked with Alzheimer’s. They also identified the types of cells that were most likely to express these disease-linked genes, and found that many of them occur most often in microglia, the immune cells responsible for clearing debris from the brain.
This study also revealed that every type of cell in the brain undergoes a phenomenon known as epigenomic erosion as Alzheimer’s disease progresses, meaning that the cells’ normal pattern of accessible genomic sites is lost, which contributes to loss of cell identity.
The role of microglia.
In a third Cell paper, led by MIT graduate student Na Sun and research scientist Matheus Victor, the researchers focused primarily on microglia, which make up 5 to 10 percent of the cells in the brain. In addition to clearing debris from the brain, these immune cells also respond to injury or infection and help neurons communicate with each other.
This study builds on a 2015 paper from Tsai and Kellis in which they found that many of the genome-wide association study (GWAS) variants associated with Alzheimer’s disease are predominantly active in immune cells like microglia, much more than in neurons or other types of brain cells.
In the new study, the researchers used RNA sequencing to classify microglia into 12 different states, based on hundreds of genes that are expressed at different levels during each state. They also showed that as Alzheimer’s disease progresses, more microglia enter inflammatory states. The Tsai lab has also previously shown that as more inflammation occurs in the brain, the blood-brain barrier begins to degrade and neurons begin to have difficulty communicating with each other.
At the same time, fewer microglia in the Alzheimer’s brain exist in a state that promotes homeostasis and helps the brain function normally. The researchers identified transcription factors that turn on the genes that keep microglia in that homeostatic state, and the Tsai lab is now exploring ways to activate those factors, in hopes of treating Alzheimer’s disease by programming inflammation-inducing microglia to switch back to a homeostatic state.
DNA damage.
In the fourth Cell study, led by MIT research scientist Vishnu Dileep and Boix, the researchers examined how DNA damage contributes to the development of Alzheimer’s disease. Previous work from Tsai’s lab has shown that DNA damage can appear in neurons long before Alzheimer’s symptoms appear. This damage is partly a consequence of the fact that during memory formation, neurons create many double-stranded DNA breaks. These breaks are promptly repaired, but the repair process can become faulty as neurons age.
This fourth study found that as more DNA damage accumulates in neurons, it becomes more difficult for them to repair the damage, leading to genome rearrangements and 3D folding defects.
“When you have a lot of DNA damage in neurons, the cells, in their attempt to put the genome back together, make mistakes that cause rearrangements,” Dileep says. “The analogy that I like to use is if you have one crack in an image, you can easily put it back together, but if you shatter an image and try to piece it back together, you’re going to make mistakes.”
These repair mistakes also lead to a phenomenon known as gene fusion, which occurs when rearrangements take place between genes, leading to dysregulation of genes. Alongside defects in genome folding, these changes appear to predominantly impact genes related to synaptic activity, likely contributing to the cognitive decline seen in Alzheimer’s disease.
The findings raise the possibility of seeking ways to enhance neurons’ DNA repair capabilities as a way to slow down the progression of Alzheimer’s disease, the researchers say.
In addition, Kellis’ lab now hopes to use artificial intelligence algorithms such as protein language models, graph neural networks, and large language models to discover drugs that might target some of the key genes that the researchers identified in these studies.
The researchers also hope that other scientists will make use of their genomic and epigenomic data. “We want the world to use this data,” Kellis says. “We've created online repositories where people can interact with the data, can access it, visualize it, and conduct analyses on the fly.”
Provided by MIT